 Found 3945 hits of ic50 data for polymerid = 1673
Found 3945 hits of ic50 data for polymerid = 1673 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 00.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D using GKPILFFRLK(DNP)-D-RNH2) labeled MCA as substrate preincubated for 10 mins followed by substrate addition measur... |
J Nat Prod 81: 1673-1681 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00417
BindingDB Entry DOI: 10.7270/Q2MW2KQC |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50400214
(CHEMBL2181022)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)N(C)C(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)N(C)C(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C55H76N8O12/c1-34(2)29-41(49(67)57-36(5)51(69)62(7)44(31-38-21-14-10-15-22-38)52(70)63-28-18-25-43(63)54(72)74-8)58-47(66)32-45(64)40(30-37-19-12-9-13-20-37)59-50(68)42(26-27-46(56)65)61(6)53(71)48(35(3)4)60-55(73)75-33-39-23-16-11-17-24-39/h9-17,19-24,34-36,40-45,48,64H,18,25-33H2,1-8H3,(H2,56,65)(H,57,67)(H,58,66)(H,59,68)(H,60,73)/t36-,40-,41-,42-,43-,44+,45-,48-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0783 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116646
BindingDB Entry DOI: 10.7270/Q2M330VK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50400214

(CHEMBL2181022)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)N(C)C(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)N(C)C(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C55H76N8O12/c1-34(2)29-41(49(67)57-36(5)51(69)62(7)44(31-38-21-14-10-15-22-38)52(70)63-28-18-25-43(63)54(72)74-8)58-47(66)32-45(64)40(30-37-19-12-9-13-20-37)59-50(68)42(26-27-46(56)65)61(6)53(71)48(35(3)4)60-55(73)75-33-39-23-16-11-17-24-39/h9-17,19-24,34-36,40-45,48,64H,18,25-33H2,1-8H3,(H2,56,65)(H,57,67)(H,58,66)(H,59,68)(H,60,73)/t36-,40-,41-,42-,43-,44+,45-,48-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0783 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D by fluorescence assay |
J Med Chem 55: 10749-65 (2012)
Article DOI: 10.1021/jm301630s
BindingDB Entry DOI: 10.7270/Q2TH8NVB |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of secreted cathepsin D in human MDA-MB-231 cells using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated... |
J Nat Prod 80: 2969-2986 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00551
BindingDB Entry DOI: 10.7270/Q2JS9SXJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50400213

(CHEMBL2181024)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)N(C)C(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)N(C)C(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C |r| Show InChI InChI=1S/C52H78N8O12/c1-31(2)27-37(45(64)54-33(5)47(66)59(10)40(29-35-21-16-13-17-22-35)48(67)60-26-18-23-39(60)50(69)71-11)55-43(63)30-41(61)36(28-34-19-14-12-15-20-34)56-46(65)38(24-25-42(53)62)58(9)49(68)44(32(3)4)57-51(70)72-52(6,7)8/h12-17,19-22,31-33,36-41,44,61H,18,23-30H2,1-11H3,(H2,53,62)(H,54,64)(H,55,63)(H,56,65)(H,57,70)/t33-,36-,37-,38-,39-,40+,41-,44-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D by fluorescence assay |
J Med Chem 55: 10749-65 (2012)
Article DOI: 10.1021/jm301630s
BindingDB Entry DOI: 10.7270/Q2TH8NVB |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.173 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50603524
(CHEMBL5192597)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccc(O)cc1)N(C)C(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)N(C)C(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.178 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116646
BindingDB Entry DOI: 10.7270/Q2M330VK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin D using Mca-Gly-Lys-Pro-Ile-Leu-PhePhe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate pretreated for 15 mins followed b... |
J Nat Prod 80: 2969-2986 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00551
BindingDB Entry DOI: 10.7270/Q2JS9SXJ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin D using Mca-Gly-Lys-Pro-Ile-Leu-Phe-Phe-Arg-Leu-Lys-(Dnp)-D-Arg-NH2 as substrate preincubated for 15 mins followe... |
Bioorg Med Chem 24: 3276-82 (2016)
Article DOI: 10.1016/j.bmc.2016.04.062
BindingDB Entry DOI: 10.7270/Q2QZ2CWR |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human placenta CatD using Abz-Lys-Pro-Ala-Glu-Phe-Nph-Ala-Leu as substrate preincubated for 10 mins followed by substrate addition and ... |
J Med Chem 63: 1576-1596 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01351
BindingDB Entry DOI: 10.7270/Q24Q7Z8X |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50377588
(CHEMBL257594)Show InChI InChI=1S/C22H19N3O2/c1-13-11-12-17-20(14(13)2)23-18-9-5-3-7-15(18)21(17)24-25-22(27)16-8-4-6-10-19(16)26/h3-12,26H,1-2H3,(H,23,24)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D by FRET assay |
Bioorg Med Chem Lett 18: 3011-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.060
BindingDB Entry DOI: 10.7270/Q2TT4RV5 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D by FRET assay |
Bioorg Med Chem Lett 18: 3011-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.060
BindingDB Entry DOI: 10.7270/Q2TT4RV5 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50506541
(CHEMBL4576166)Show SMILES CC(C)[C@@H]1NC(=O)C[C@H](O)[C@H](Cc2ccc3ccccc3c2)NC(=O)CCCCCCCCCCCNC1=O |r| Show InChI InChI=1S/C32H47N3O4/c1-23(2)31-32(39)33-19-13-9-7-5-3-4-6-8-10-16-29(37)34-27(28(36)22-30(38)35-31)21-24-17-18-25-14-11-12-15-26(25)20-24/h11-12,14-15,17-18,20,23,27-28,31,36H,3-10,13,16,19,21-22H2,1-2H3,(H,33,39)(H,34,37)(H,35,38)/t27-,28-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human placenta CatD using Abz-Lys-Pro-Ala-Glu-Phe-Nph-Ala-Leu as substrate preincubated for 10 mins followed by substrate addition and ... |
J Med Chem 63: 1576-1596 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01351
BindingDB Entry DOI: 10.7270/Q24Q7Z8X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin D
(Homo sapiens (Human)) | BDBM50312541
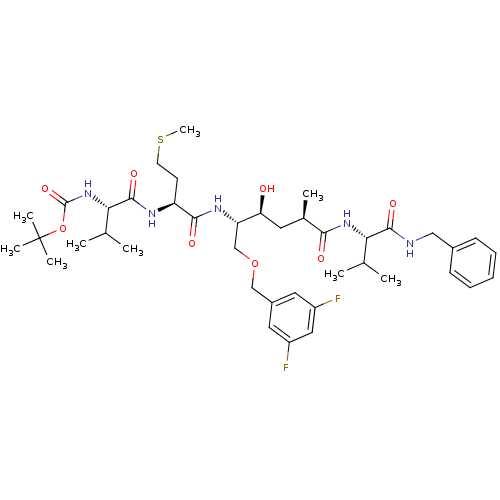
(((S)-1-[(S)-1-[(1S,2S,4R)-4-((S)-1-Benzylcarbamoyl...)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C)C(=O)N[C@@H](COCc1cc(F)cc(F)c1)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C41H61F2N5O8S/c1-24(2)34(38(52)44-21-27-13-11-10-12-14-27)47-36(50)26(5)17-33(49)32(23-55-22-28-18-29(42)20-30(43)19-28)46-37(51)31(15-16-57-9)45-39(53)35(25(3)4)48-40(54)56-41(6,7)8/h10-14,18-20,24-26,31-35,49H,15-17,21-23H2,1-9H3,(H,44,52)(H,45,53)(H,46,51)(H,47,50)(H,48,54)/t26-,31+,32+,33+,34+,35+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Link£ping University
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin D after 20 mins by homogeneous time resolved fluorescence assay |
Eur J Med Chem 45: 870-82 (2010)
Article DOI: 10.1016/j.ejmech.2009.11.013
BindingDB Entry DOI: 10.7270/Q2ZG6SCV |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.693 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D by fluorescence assay |
J Med Chem 55: 10749-65 (2012)
Article DOI: 10.1021/jm301630s
BindingDB Entry DOI: 10.7270/Q2TH8NVB |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 0.693 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116646
BindingDB Entry DOI: 10.7270/Q2M330VK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50080960
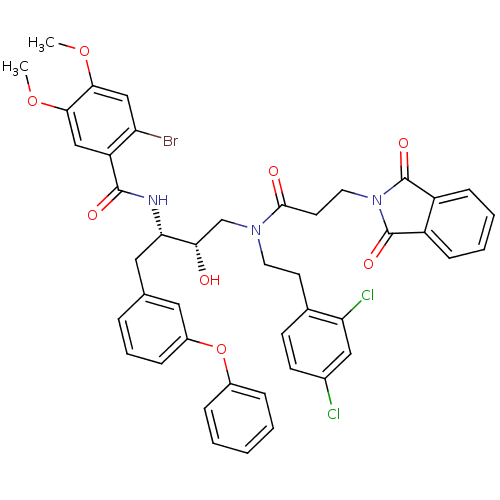
(2-Bromo-N-[(1S,2S)-3-{[2-(2,4-dichloro-phenyl)-eth...)Show SMILES COc1cc(Br)c(cc1OC)C(=O)N[C@@H](Cc1cccc(Oc2ccccc2)c1)[C@@H](O)CN(CCc1ccc(Cl)cc1Cl)C(=O)CCN1C(=O)c2ccccc2C1=O Show InChI InChI=1S/C44H40BrCl2N3O8/c1-56-39-24-34(35(45)25-40(39)57-2)42(53)48-37(22-27-9-8-12-31(21-27)58-30-10-4-3-5-11-30)38(51)26-49(19-17-28-15-16-29(46)23-36(28)47)41(52)18-20-50-43(54)32-13-6-7-14-33(32)44(50)55/h3-16,21,23-25,37-38,51H,17-20,22,26H2,1-2H3,(H,48,53)/t37-,38-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human liver Cathepsin D using Cathepsin D assay. |
Bioorg Med Chem Lett 9: 2531-6 (1999)
BindingDB Entry DOI: 10.7270/Q25H7GRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin D
(Homo sapiens (Human)) | BDBM50437552
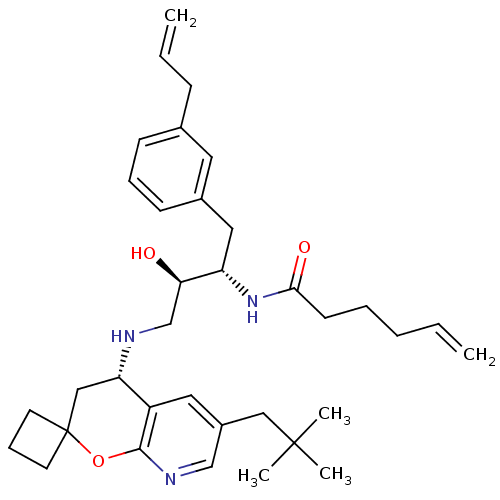
(CHEMBL2407339)Show SMILES CC(C)(C)Cc1cnc2OC3(CCC3)C[C@H](NC[C@@H](O)[C@H](Cc3cccc(CC=C)c3)NC(=O)CCCC=C)c2c1 |r| Show InChI InChI=1S/C35H49N3O3/c1-6-8-9-15-32(40)38-29(20-26-14-10-13-25(18-26)12-7-2)31(39)24-36-30-22-35(16-11-17-35)41-33-28(30)19-27(23-37-33)21-34(3,4)5/h6-7,10,13-14,18-19,23,29-31,36,39H,1-2,8-9,11-12,15-17,20-22,24H2,3-5H3,(H,38,40)/t29-,30-,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cathepsin D (unknown origin) by FRET assay |
Bioorg Med Chem Lett 23: 4459-64 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.028
BindingDB Entry DOI: 10.7270/Q2FQ9Z1B |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Cathepsin D (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115879
BindingDB Entry DOI: 10.7270/Q2HQ43KW |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50294218
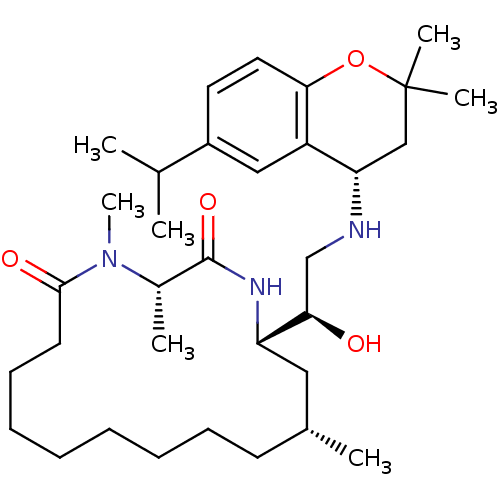
((3S,14R,16S)-16-((R)-1-hydroxy-2-((S)-6-isopropyl-...)Show SMILES CC(C)c1ccc2OC(C)(C)C[C@H](NC[C@@H](O)[C@@H]3C[C@H](C)CCCCCCCCC(=O)N(C)[C@@H](C)C(=O)N3)c2c1 |r| Show InChI InChI=1S/C33H55N3O4/c1-22(2)25-16-17-30-26(19-25)28(20-33(5,6)40-30)34-21-29(37)27-18-23(3)14-12-10-8-9-11-13-15-31(38)36(7)24(4)32(39)35-27/h16-17,19,22-24,27-29,34,37H,8-15,18,20-21H2,1-7H3,(H,35,39)/t23-,24+,27+,28+,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 19: 1366-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.055
BindingDB Entry DOI: 10.7270/Q2SB45S3 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D (unknown origin) |
Bioorg Med Chem Lett 24: 4141-50 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.054
BindingDB Entry DOI: 10.7270/Q2W95BV1 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM232262

(US9345742, 20)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](N)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC |r| Show InChI InChI=1S/C42H64N6O8/c1-11-27(6)36(39(52)48-35(26(4)5)40(53)55-10)46-33(49)24-30(43)31(22-28-18-14-12-15-19-28)44-38(51)34(25(2)3)47-37(50)32(23-29-20-16-13-17-21-29)45-41(54)56-42(7,8)9/h12-21,25-27,30-32,34-36H,11,22-24,43H2,1-10H3,(H,44,51)(H,45,54)(H,46,49)(H,47,50)(H,48,52)/t27-,30-,31-,32-,34-,35-,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 5.5 | 25 |
MERCK PATENT GMBH
US Patent
| Assay Description
In order to identify modulators of cathepsin D activity, a continuous enzymatic test was carried out with a synthetic peptide which carries a fluores... |
US Patent US9345742 (2016)
BindingDB Entry DOI: 10.7270/Q25Q4TZD |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50305527
((4S)-4-[(1R)-1-hydroxy-2-({1-[3-(1-methylethyl)phe...)Show SMILES COCc1cc2NCCCCOc3cccc(C[C@H](NC(=O)c(c1)c2)[C@H](O)CNC1(CC1)c1cccc(c1)C(C)C)c3 |r| Show InChI InChI=1S/C35H45N3O4/c1-24(2)27-9-7-10-29(20-27)35(12-13-35)37-22-33(39)32-19-25-8-6-11-31(18-25)42-15-5-4-14-36-30-17-26(23-41-3)16-28(21-30)34(40)38-32/h6-11,16-18,20-21,24,32-33,36-37,39H,4-5,12-15,19,22-23H2,1-3H3,(H,38,40)/t32-,33+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 603-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.092
BindingDB Entry DOI: 10.7270/Q2H1324C |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438363
(CHEMBL2408751)Show SMILES CCC[C@@]1(C)C[C@H](NC[C@@H](O)[C@H](Cc2cc(F)cc(F)c2)NC(C)=O)c2cc(CC(C)(C)C)ccc2O1 |r| Show InChI InChI=1S/C30H42F2N2O3/c1-7-10-30(6)17-26(24-13-20(16-29(3,4)5)8-9-28(24)37-30)33-18-27(36)25(34-19(2)35)14-21-11-22(31)15-23(32)12-21/h8-9,11-13,15,25-27,33,36H,7,10,14,16-18H2,1-6H3,(H,34,35)/t25-,26-,27+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50506521
(CHEMBL4558691)Show SMILES O[C@H]1CC(=O)N[C@@H](Cc2cccnc2)C(=O)NCCCCCCCCCCCC(=O)N[C@H]1Cc1ccccc1 |r| Show InChI InChI=1S/C31H44N4O4/c36-28-22-30(38)35-27(21-25-16-13-18-32-23-25)31(39)33-19-12-7-5-3-1-2-4-6-11-17-29(37)34-26(28)20-24-14-9-8-10-15-24/h8-10,13-16,18,23,26-28,36H,1-7,11-12,17,19-22H2,(H,33,39)(H,34,37)(H,35,38)/t26-,27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human placenta CatD using Abz-Lys-Pro-Ala-Glu-Phe-Nph-Ala-Leu as substrate preincubated for 10 mins followed by substrate addition and ... |
J Med Chem 63: 1576-1596 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01351
BindingDB Entry DOI: 10.7270/Q24Q7Z8X |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50383837
(CHEMBL2030998)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C28H37F2N3O3/c1-17(34)33-23(11-18-8-20(29)12-21(30)9-18)25(35)16-31-24-14-28(6-5-7-28)36-26-22(24)10-19(15-32-26)13-27(2,3)4/h8-10,12,15,23-25,31,35H,5-7,11,13-14,16H2,1-4H3,(H,33,34)/t23-,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50438362
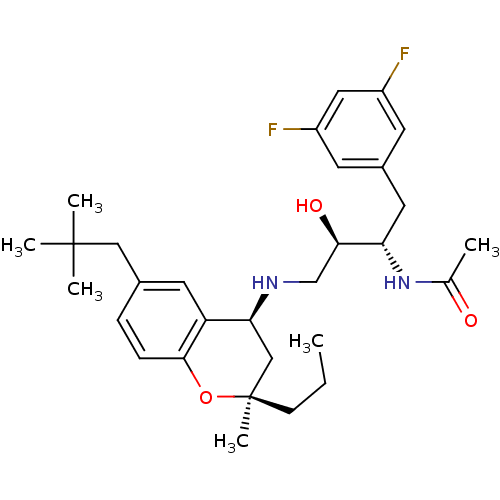
(CHEMBL2408752)Show SMILES CCC[C@]1(C)C[C@H](NC[C@@H](O)[C@H](Cc2cc(F)cc(F)c2)NC(C)=O)c2cc(CC(C)(C)C)ccc2O1 |r| Show InChI InChI=1S/C30H42F2N2O3/c1-7-10-30(6)17-26(24-13-20(16-29(3,4)5)8-9-28(24)37-30)33-18-27(36)25(34-19(2)35)14-21-11-22(31)15-23(32)12-21/h8-9,11-13,15,25-27,33,36H,7,10,14,16-18H2,1-6H3,(H,34,35)/t25-,26-,27+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin-D (unknown origin) using C-terminal biotinylated peptide substrate treated 30 mins before addition of peptide substrate measu... |
Bioorg Med Chem Lett 23: 4674-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.006
BindingDB Entry DOI: 10.7270/Q29W0GWT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against human liver Cathepsin D using Cathepsin D assay. |
Bioorg Med Chem Lett 9: 2531-6 (1999)
BindingDB Entry DOI: 10.7270/Q25H7GRN |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50437542

(CHEMBL2407492)Show SMILES CC(C)(C)Cc1cnc2OC3(CCC3)C[C@H](NC[C@@H](O)[C@@H]3Cc4cccc(CCCn5cc(ccc5=O)C(=O)N3)c4)c2c1 |r| Show InChI InChI=1S/C35H44N4O4/c1-34(2,3)18-25-16-27-29(19-35(12-6-13-35)43-33(27)37-20-25)36-21-30(40)28-17-24-8-4-7-23(15-24)9-5-14-39-22-26(32(42)38-28)10-11-31(39)41/h4,7-8,10-11,15-16,20,22,28-30,36,40H,5-6,9,12-14,17-19,21H2,1-3H3,(H,38,42)/t28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cathepsin D (unknown origin) by FRET assay |
Bioorg Med Chem Lett 23: 4459-64 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.028
BindingDB Entry DOI: 10.7270/Q2FQ9Z1B |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal and Aromatic Plants
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 16: 4603-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.010
BindingDB Entry DOI: 10.7270/Q2FB53QN |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
| n/a | n/a | 2.33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of cathepsin D (unknown origin) using hemoglobin as substrate after 30 min by spectrophotometric analysis |
Citation and Details
Article DOI: 10.1007/s00044-012-0397-z
BindingDB Entry DOI: 10.7270/Q29026PM |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50506530
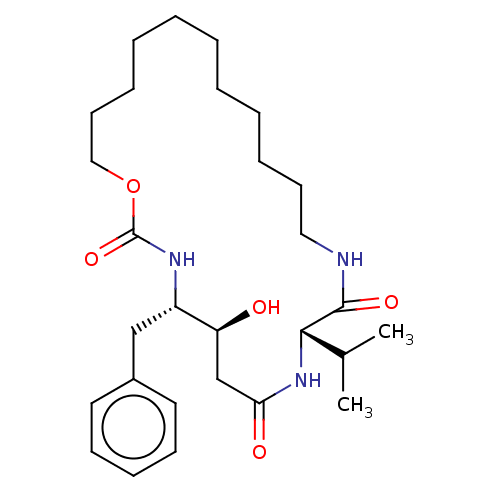
(CHEMBL4514021)Show SMILES CC(C)[C@@H]1NC(=O)C[C@H](O)[C@H](Cc2ccccc2)NC(=O)OCCCCCCCCCCCNC1=O |r| Show InChI InChI=1S/C28H45N3O5/c1-21(2)26-27(34)29-17-13-8-6-4-3-5-7-9-14-18-36-28(35)30-23(24(32)20-25(33)31-26)19-22-15-11-10-12-16-22/h10-12,15-16,21,23-24,26,32H,3-9,13-14,17-20H2,1-2H3,(H,29,34)(H,30,35)(H,31,33)/t23-,24-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human placenta CatD using Abz-Lys-Pro-Ala-Glu-Phe-Nph-Ala-Leu as substrate preincubated for 10 mins followed by substrate addition and ... |
J Med Chem 63: 1576-1596 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01351
BindingDB Entry DOI: 10.7270/Q24Q7Z8X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin D
(Homo sapiens (Human)) | BDBM50383839
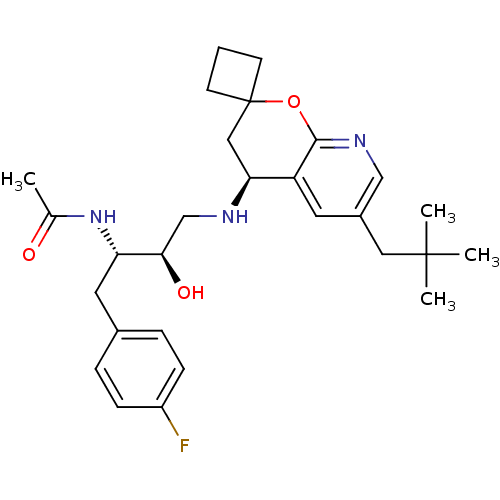
(CHEMBL2030997)Show SMILES CC(=O)N[C@@H](Cc1ccc(F)cc1)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C28H38FN3O3/c1-18(33)32-23(13-19-6-8-21(29)9-7-19)25(34)17-30-24-15-28(10-5-11-28)35-26-22(24)12-20(16-31-26)14-27(2,3)4/h6-9,12,16,23-25,30,34H,5,10-11,13-15,17H2,1-4H3,(H,32,33)/t23-,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50305536
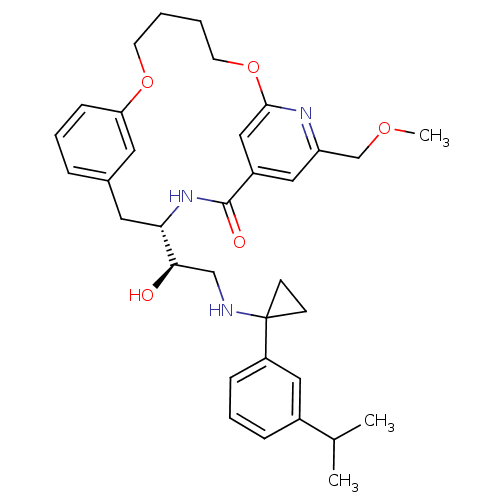
((S)-4-{(R)-1-Hydroxy-2-[1-(3-isopropyl-phenyl)-cyc...)Show SMILES COCc1cc2cc(OCCCCOc3cccc(C[C@H](NC2=O)[C@H](O)CNC2(CC2)c2cccc(c2)C(C)C)c3)n1 |r| Show InChI InChI=1S/C34H43N3O5/c1-23(2)25-9-7-10-27(18-25)34(12-13-34)35-21-31(38)30-17-24-8-6-11-29(16-24)41-14-4-5-15-42-32-20-26(33(39)37-30)19-28(36-32)22-40-3/h6-11,16,18-20,23,30-31,35,38H,4-5,12-15,17,21-22H2,1-3H3,(H,37,39)/t30-,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedicalResearch
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 20: 603-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.092
BindingDB Entry DOI: 10.7270/Q2H1324C |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50506516
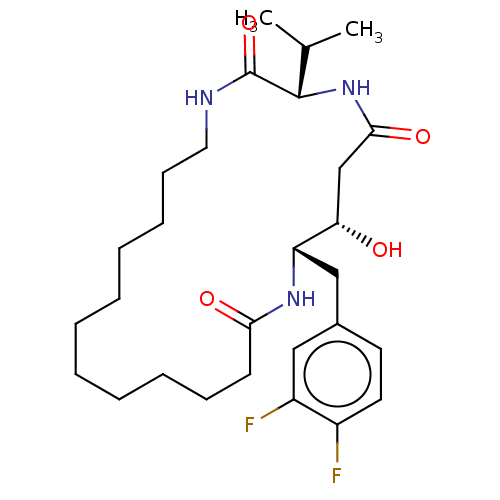
(CHEMBL4444244)Show SMILES CC(C)[C@@H]1NC(=O)C[C@H](O)[C@H](Cc2ccc(F)c(F)c2)NC(=O)CCCCCCCCCCCNC1=O |r| Show InChI InChI=1S/C28H43F2N3O4/c1-19(2)27-28(37)31-15-11-9-7-5-3-4-6-8-10-12-25(35)32-23(24(34)18-26(36)33-27)17-20-13-14-21(29)22(30)16-20/h13-14,16,19,23-24,27,34H,3-12,15,17-18H2,1-2H3,(H,31,37)(H,32,35)(H,33,36)/t23-,24-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human placenta CatD using Abz-Lys-Pro-Ala-Glu-Phe-Nph-Ala-Leu as substrate preincubated for 10 mins followed by substrate addition and ... |
J Med Chem 63: 1576-1596 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01351
BindingDB Entry DOI: 10.7270/Q24Q7Z8X |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM232279

(US9345742, 37)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](N)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)Cc1cccc(OC(F)(F)F)c1)[C@@H](C)CC)C(=O)N[C@@H](C(C)C)C(O)=O |r| Show InChI InChI=1S/C37H52F3N5O7/c1-7-22(5)32(43-29(46)19-25-15-12-16-26(17-25)52-37(38,39)40)34(48)42-28(18-24-13-10-9-11-14-24)27(41)20-30(47)44-33(23(6)8-2)35(49)45-31(21(3)4)36(50)51/h9-17,21-23,27-28,31-33H,7-8,18-20,41H2,1-6H3,(H,42,48)(H,43,46)(H,44,47)(H,45,49)(H,50,51)/t22-,23-,27-,28-,31-,32-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 5.5 | 25 |
MERCK PATENT GMBH
US Patent
| Assay Description
In order to identify modulators of cathepsin D activity, a continuous enzymatic test was carried out with a synthetic peptide which carries a fluores... |
US Patent US9345742 (2016)
BindingDB Entry DOI: 10.7270/Q25Q4TZD |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50302846
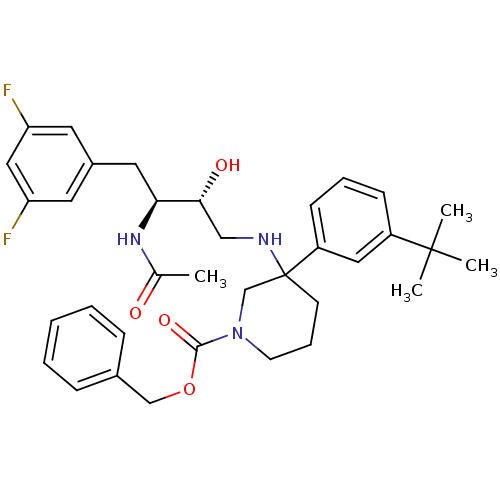
(CHEMBL570165 | benzyl 3-((2R,3S)-3-acetamido-4-(3,...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCN(C1)C(=O)OCc1ccccc1)c1cccc(c1)C(C)(C)C |r| Show InChI InChI=1S/C35H43F2N3O4/c1-24(41)39-31(18-26-16-29(36)20-30(37)17-26)32(42)21-38-35(28-13-8-12-27(19-28)34(2,3)4)14-9-15-40(23-35)33(43)44-22-25-10-6-5-7-11-25/h5-8,10-13,16-17,19-20,31-32,38,42H,9,14-15,18,21-23H2,1-4H3,(H,39,41)/t31-,32+,35?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D assessed as reduction in polarization after 110 mins by oregon green based fluorescence polarization assay |
Bioorg Med Chem Lett 19: 6386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.061
BindingDB Entry DOI: 10.7270/Q23779NV |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50506535
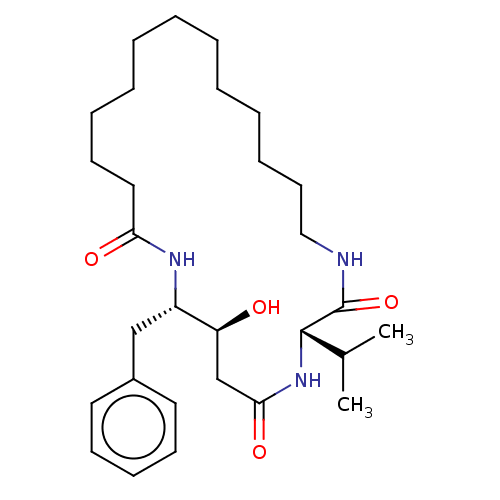
(CHEMBL4560400)Show SMILES CC(C)[C@@H]1NC(=O)C[C@H](O)[C@H](Cc2ccccc2)NC(=O)CCCCCCCCCCCCNC1=O |r| Show InChI InChI=1S/C29H47N3O4/c1-22(2)28-29(36)30-19-15-10-8-6-4-3-5-7-9-14-18-26(34)31-24(25(33)21-27(35)32-28)20-23-16-12-11-13-17-23/h11-13,16-17,22,24-25,28,33H,3-10,14-15,18-21H2,1-2H3,(H,30,36)(H,31,34)(H,32,35)/t24-,25-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human placenta CatD using Abz-Lys-Pro-Ala-Glu-Phe-Nph-Ala-Leu as substrate preincubated for 10 mins followed by substrate addition and ... |
J Med Chem 63: 1576-1596 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01351
BindingDB Entry DOI: 10.7270/Q24Q7Z8X |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM232284
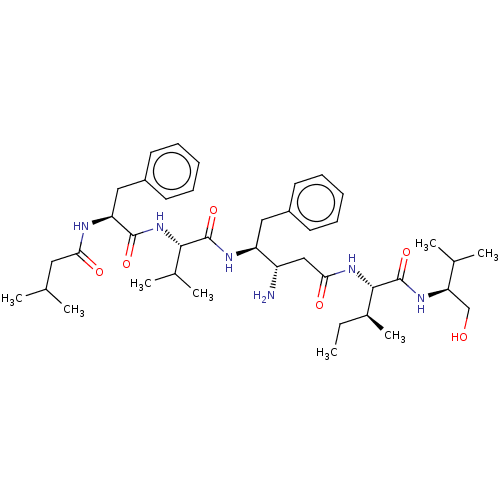
(US9345742, 42)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](N)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(C)C)C(=O)N[C@H](CO)C(C)C |r| Show InChI InChI=1S/C41H64N6O6/c1-9-28(8)38(41(53)45-34(24-48)26(4)5)46-36(50)23-31(42)32(21-29-16-12-10-13-17-29)44-40(52)37(27(6)7)47-39(51)33(43-35(49)20-25(2)3)22-30-18-14-11-15-19-30/h10-19,25-28,31-34,37-38,48H,9,20-24,42H2,1-8H3,(H,43,49)(H,44,52)(H,45,53)(H,46,50)(H,47,51)/t28-,31-,32-,33-,34+,37-,38-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 5.5 | 25 |
MERCK PATENT GMBH
US Patent
| Assay Description
In order to identify modulators of cathepsin D activity, a continuous enzymatic test was carried out with a synthetic peptide which carries a fluores... |
US Patent US9345742 (2016)
BindingDB Entry DOI: 10.7270/Q25Q4TZD |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM232270

(US9345742, 28)Show SMILES COC(=O)[C@@H](NC(=O)[C@H](Cc1cccc2ccccc12)NC(=O)C[C@H](N)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)CC(C)C)C(C)C)C(C)C |r| Show InChI InChI=1S/C46H66N6O7/c1-27(2)22-37(48-39(53)23-28(3)4)43(55)51-41(29(5)6)45(57)50-36(24-31-16-11-10-12-17-31)35(47)26-40(54)49-38(44(56)52-42(30(7)8)46(58)59-9)25-33-20-15-19-32-18-13-14-21-34(32)33/h10-21,27-30,35-38,41-42H,22-26,47H2,1-9H3,(H,48,53)(H,49,54)(H,50,57)(H,51,55)(H,52,56)/t35-,36-,37-,38-,41-,42-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 5.5 | 25 |
MERCK PATENT GMBH
US Patent
| Assay Description
In order to identify modulators of cathepsin D activity, a continuous enzymatic test was carried out with a synthetic peptide which carries a fluores... |
US Patent US9345742 (2016)
BindingDB Entry DOI: 10.7270/Q25Q4TZD |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50506522
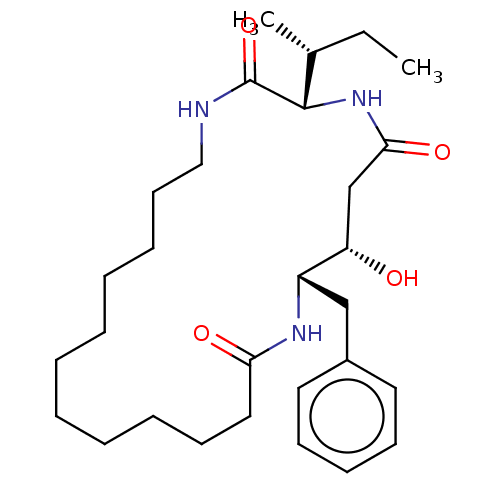
(CHEMBL4532529)Show SMILES [H][C@]1(NC(=O)C[C@H](O)[C@H](Cc2ccccc2)NC(=O)CCCCCCCCCCCNC1=O)[C@H](C)CC |r| Show InChI InChI=1S/C29H47N3O4/c1-3-22(2)28-29(36)30-19-15-10-8-6-4-5-7-9-14-18-26(34)31-24(25(33)21-27(35)32-28)20-23-16-12-11-13-17-23/h11-13,16-17,22,24-25,28,33H,3-10,14-15,18-21H2,1-2H3,(H,30,36)(H,31,34)(H,32,35)/t22-,24+,25+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human placenta CatD using Abz-Lys-Pro-Ala-Glu-Phe-Nph-Ala-Leu as substrate preincubated for 10 mins followed by substrate addition and ... |
J Med Chem 63: 1576-1596 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01351
BindingDB Entry DOI: 10.7270/Q24Q7Z8X |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM232261
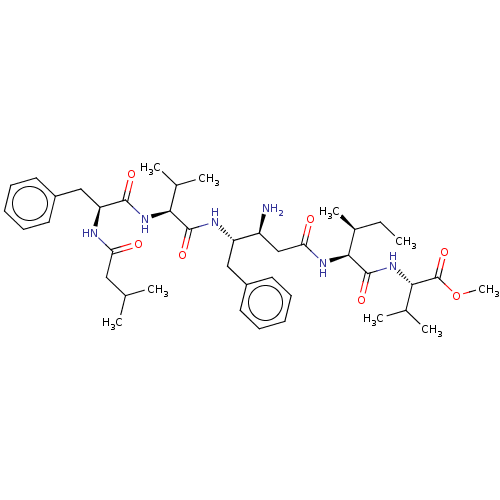
(US9345742, 19)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](N)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(C)C)C(=O)N[C@@H](C(C)C)C(=O)OC |r| Show InChI InChI=1S/C42H64N6O7/c1-10-28(8)38(41(53)48-37(27(6)7)42(54)55-9)46-35(50)24-31(43)32(22-29-17-13-11-14-18-29)45-40(52)36(26(4)5)47-39(51)33(44-34(49)21-25(2)3)23-30-19-15-12-16-20-30/h11-20,25-28,31-33,36-38H,10,21-24,43H2,1-9H3,(H,44,49)(H,45,52)(H,46,50)(H,47,51)(H,48,53)/t28-,31-,32-,33-,36-,37-,38-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 5.5 | 25 |
MERCK PATENT GMBH
US Patent
| Assay Description
In order to identify modulators of cathepsin D activity, a continuous enzymatic test was carried out with a synthetic peptide which carries a fluores... |
US Patent US9345742 (2016)
BindingDB Entry DOI: 10.7270/Q25Q4TZD |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50555173

(CHEMBL4754691)Show SMILES OC(=O)C(F)(F)F.CNS(=O)(=O)c1ccc(C)c(NC(=O)[C@@H](CC2CCCCC2)NC(=N)NC(=O)Cc2ccc(OC)c(OC)c2)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver Cathepsin D preincubated for 10 mins followed by substrate addition and further incubated for 2 hrs in dark by fluorimetric... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115879
BindingDB Entry DOI: 10.7270/Q2HQ43KW |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50383838
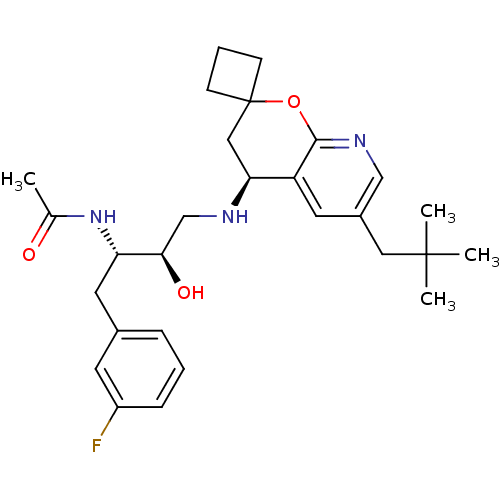
(CHEMBL2030996)Show SMILES CC(=O)N[C@@H](Cc1cccc(F)c1)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C28H38FN3O3/c1-18(33)32-23(13-19-7-5-8-21(29)11-19)25(34)17-30-24-15-28(9-6-10-28)35-26-22(24)12-20(16-31-26)14-27(2,3)4/h5,7-8,11-12,16,23-25,30,34H,6,9-10,13-15,17H2,1-4H3,(H,32,33)/t23-,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50383846
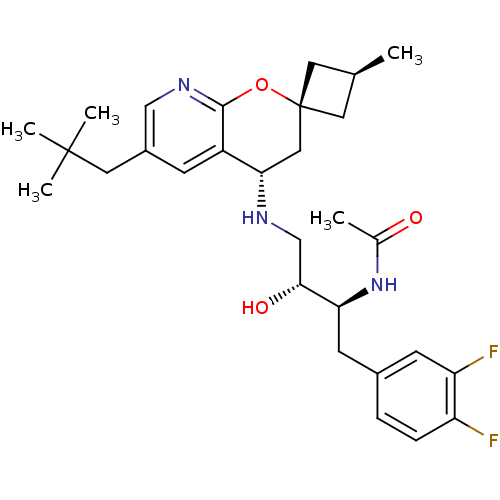
(CHEMBL2031142)Show SMILES C[C@H]1C[C@]2(C1)C[C@H](NC[C@@H](O)[C@H](Cc1ccc(F)c(F)c1)NC(C)=O)c1cc(CC(C)(C)C)cnc1O2 |r,wU:6.7,1.0,wD:9.10,11.22,3.3,(31.56,-19.68,;30.47,-20.77,;30.47,-22.31,;28.93,-22.31,;28.93,-20.77,;27.59,-21.54,;26.25,-22.32,;24.91,-21.56,;23.58,-22.33,;22.25,-21.57,;22.24,-20.03,;20.91,-22.34,;20.92,-23.88,;19.59,-24.66,;19.61,-26.19,;18.28,-26.97,;16.93,-26.21,;15.61,-26.99,;16.93,-24.67,;15.59,-23.9,;18.26,-23.89,;19.58,-21.58,;19.57,-20.04,;18.23,-19.28,;20.9,-19.26,;26.26,-23.86,;24.93,-24.63,;24.93,-26.18,;23.6,-26.95,;23.6,-28.49,;22.26,-29.25,;24.93,-29.26,;23.58,-30.02,;26.27,-26.95,;27.6,-26.18,;27.6,-24.63,;28.93,-23.86,)| Show InChI InChI=1S/C29H39F2N3O3/c1-17-11-29(12-17)14-25(21-8-20(13-28(3,4)5)15-33-27(21)37-29)32-16-26(36)24(34-18(2)35)10-19-6-7-22(30)23(31)9-19/h6-9,15,17,24-26,32,36H,10-14,16H2,1-5H3,(H,34,35)/t17-,24-,25-,26+,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Envoy Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D |
Bioorg Med Chem Lett 22: 3607-11 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.060
BindingDB Entry DOI: 10.7270/Q2BP03TM |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM232275
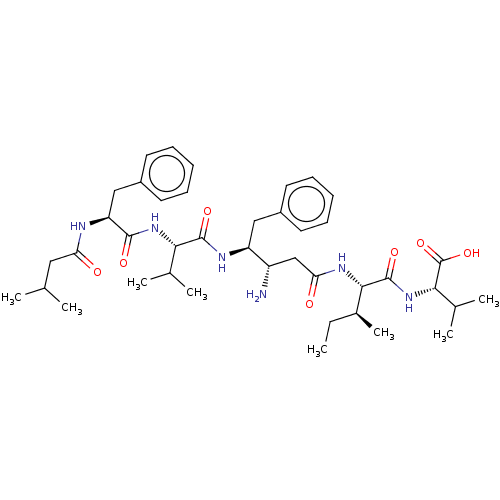
(US9345742, 33)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](N)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(C)C)C(=O)N[C@@H](C(C)C)C(O)=O |r| Show InChI InChI=1S/C41H62N6O7/c1-9-27(8)37(40(52)47-36(26(6)7)41(53)54)45-34(49)23-30(42)31(21-28-16-12-10-13-17-28)44-39(51)35(25(4)5)46-38(50)32(43-33(48)20-24(2)3)22-29-18-14-11-15-19-29/h10-19,24-27,30-32,35-37H,9,20-23,42H2,1-8H3,(H,43,48)(H,44,51)(H,45,49)(H,46,50)(H,47,52)(H,53,54)/t27-,30-,31-,32-,35-,36-,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | 5.5 | 25 |
MERCK PATENT GMBH
US Patent
| Assay Description
In order to identify modulators of cathepsin D activity, a continuous enzymatic test was carried out with a synthetic peptide which carries a fluores... |
US Patent US9345742 (2016)
BindingDB Entry DOI: 10.7270/Q25Q4TZD |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50506517
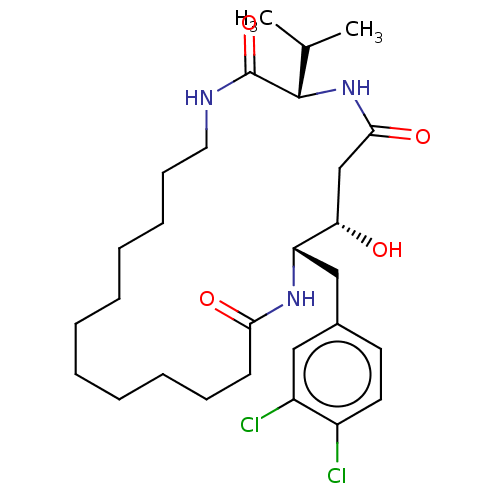
(CHEMBL4569563)Show SMILES CC(C)[C@@H]1NC(=O)C[C@H](O)[C@H](Cc2ccc(Cl)c(Cl)c2)NC(=O)CCCCCCCCCCCNC1=O |r| Show InChI InChI=1S/C28H43Cl2N3O4/c1-19(2)27-28(37)31-15-11-9-7-5-3-4-6-8-10-12-25(35)32-23(24(34)18-26(36)33-27)17-20-13-14-21(29)22(30)16-20/h13-14,16,19,23-24,27,34H,3-12,15,17-18H2,1-2H3,(H,31,37)(H,32,35)(H,33,36)/t23-,24-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human placenta CatD using Abz-Lys-Pro-Ala-Glu-Phe-Nph-Ala-Leu as substrate preincubated for 10 mins followed by substrate addition and ... |
J Med Chem 63: 1576-1596 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01351
BindingDB Entry DOI: 10.7270/Q24Q7Z8X |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50437546

(CHEMBL2407487)Show SMILES CC(C)(C)Cc1cnc2OC3(CCC3)C[C@H](NC[C@@H](O)[C@@H]3Cc4cccc(CCCCCCC(=O)N3)c4)c2c1 |r| Show InChI InChI=1S/C33H47N3O3/c1-32(2,3)19-25-17-26-28(20-33(14-9-15-33)39-31(26)35-21-25)34-22-29(37)27-18-24-12-8-11-23(16-24)10-6-4-5-7-13-30(38)36-27/h8,11-12,16-17,21,27-29,34,37H,4-7,9-10,13-15,18-20,22H2,1-3H3,(H,36,38)/t27-,28-,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cathepsin D (unknown origin) by FRET assay |
Bioorg Med Chem Lett 23: 4459-64 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.028
BindingDB Entry DOI: 10.7270/Q2FQ9Z1B |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50392776

(CHEMBL2151151)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@H]1CC2(CCC2)Oc2ncc(CC(C)(C)C)cc12 |r| Show InChI InChI=1S/C28H39N3O3/c1-19(32)31-23(14-20-9-6-5-7-10-20)25(33)18-29-24-16-28(11-8-12-28)34-26-22(24)13-21(17-30-26)15-27(2,3)4/h5-7,9-10,13,17,23-25,29,33H,8,11-12,14-16,18H2,1-4H3,(H,31,32)/t23-,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Cathepsin D (unknown origin) by FRET assay |
Bioorg Med Chem Lett 23: 4459-64 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.028
BindingDB Entry DOI: 10.7270/Q2FQ9Z1B |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50506518
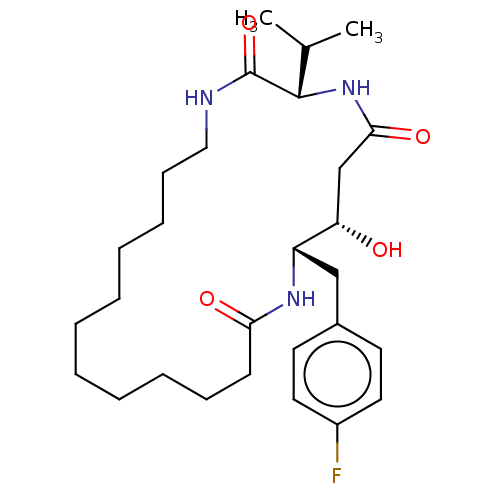
(CHEMBL4551750)Show SMILES CC(C)[C@@H]1NC(=O)C[C@H](O)[C@H](Cc2ccc(F)cc2)NC(=O)CCCCCCCCCCCNC1=O |r| Show InChI InChI=1S/C28H44FN3O4/c1-20(2)27-28(36)30-17-11-9-7-5-3-4-6-8-10-12-25(34)31-23(24(33)19-26(35)32-27)18-21-13-15-22(29)16-14-21/h13-16,20,23-24,27,33H,3-12,17-19H2,1-2H3,(H,30,36)(H,31,34)(H,32,35)/t23-,24-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Czech Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human placenta CatD using Abz-Lys-Pro-Ala-Glu-Phe-Nph-Ala-Leu as substrate preincubated for 10 mins followed by substrate addition and ... |
J Med Chem 63: 1576-1596 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01351
BindingDB Entry DOI: 10.7270/Q24Q7Z8X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data